HARD
JEE Main/Advance
IMPORTANT
Earn 100
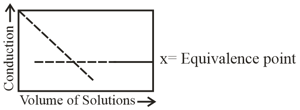
The following curve for the conductometric titration is obtained when -

(a)solution is added into solution.
(b)solution is added into solution.
(c) solution is added into solution.
(d) solution is added into solution.
100% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
What is the at for the cell, .
The standard reduction potentials for the half-reactions and are and respectively.
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Two weak acid solutions and each with the same concentration and having values and are placed in contact with hydrogen electrode (,) and are interconnected through a salt bridge. The of the cell is:
MEDIUM
JEE Main/Advance
IMPORTANT
