EASY
NEET
IMPORTANT
Earn 100
mole of an ideal gas expands isothermally and reversibly from to at . What is the internal energy change:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
thermodynamic system goes in cyclic reversible process as represented in the following diagram:
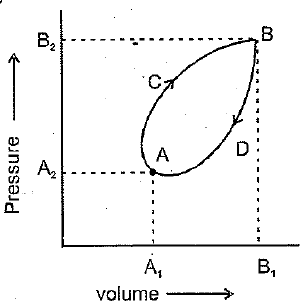
The net work done during the complete cycle is given by the area :
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
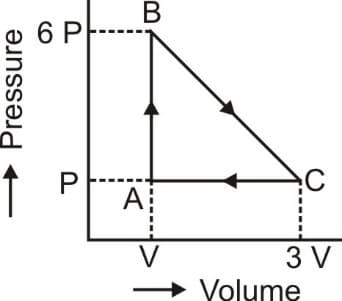
EASY
NEET
IMPORTANT
In cyclic process, the magnitude of work done is:-
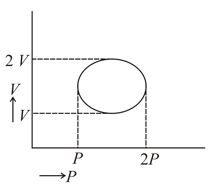
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
