A gas described by van der Waals equation (multiple correct answers type)

Important Questions on Miscellaneous Objective Questions
A solution is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture is .
Given,
Standard freezing point of water
Standard freezing point of ethanol
Standard boiling point of water
Standard boiling point of ethanol
Vapour pressure of pure water
Vapour pressure of pure ethanol
Molecular weight of water
Molecular weight of ethanol
The freezing point of the solution is
A solution is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture is .
Given,
Standard freezing point of water
Standard freezing point of ethanol
Standard boiling point of water
Standard boiling point of ethanol
Vapour pressure of pure water
Vapour pressure of pure ethanol
Molecular weight of water
Molecular weight of ethanol
The vapour pressure of the solution is
A solution M is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture is Given
Standard freezing point of water
Standard freezing point of ethanol
Standard boiling point of water
Standard boiling point of ethanol
Vapour pressure of pure water
Vapour pressure of pure ethanol
Molecular weight of water
Molecular weight of ethanol
Water is added to the solution M such that the mole fraction of water in solution becomes 0.9. What is the boiling point of this solution?
[Hint: Solute is ethanol and solvent is water]
Electrolysis of dilute aqueous solution was carried out by passing a current of . The time required to liberate of gas at the cathode is .
How many effective numbers of atoms (spheres) are contained within the hexagonal prism shown below in the space lattice?
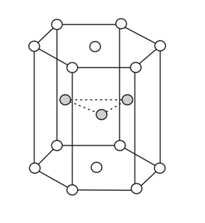
The cell content of the unit cell of a space lattice is
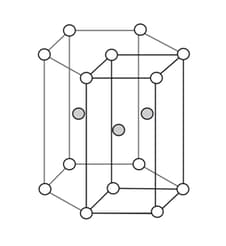
The volume of the hexagonal prism is (radius of every sphere is $r)$