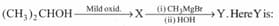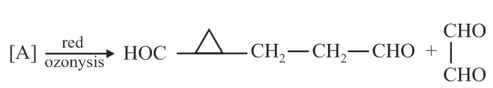HARD
JEE Advanced
IMPORTANT
Earn 100
An aromatic compound with molecular formula with gives moles of and
mole of Both and give yellow precipitate with with gives
and moles of . Compound with red and gives . Which has zero dipole moment? Give the structures of and .

Important Questions on Reduction and Oxidation Reactions of Organic Compounds
HARD
JEE Advanced
IMPORTANT
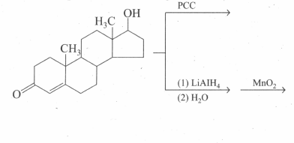
An optically active alcohol absorbs two moles of hydrogen per mole of . Upon catalytic hydrogenation, gives a product . The compound is resistant to oxidation by and does not show any optical activity. Deduce the structures of and
HARD
JEE Advanced
IMPORTANT
An unknown hydrocarbon , with formula , react with molar equivalent of over a palladium catalyst. Hydrocarbon also react with to give a diol . When oxidized with in acidic solution, '' gives two fragments. One fragment is propanoic acid and other fragment is a ketone . What are the structures of and ?
HARD
JEE Advanced
IMPORTANT
Predict the products of the following reaction:
HARD
JEE Advanced
IMPORTANT

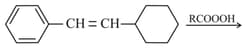
The product is
HARD
JEE Advanced
IMPORTANT
An unknown compound decolorizes bromine in carbon tetrachloride, and it undergoes catalytic reduction to give decalin. When treated with warm, concentrated potassium permanganate, this compound gives -cyclohexanedicarboxylic acid and oxalic acid. A possible structure for the unknown compound is: