MEDIUM
JEE Main
IMPORTANT
Earn 100
Compound (molar mass ) containing carbon, hydrogen and oxygen gives a reddish-brown precipitate in Fehling's test. is?
(a)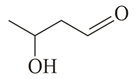

(b)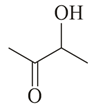

(c)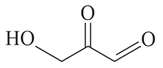

(d)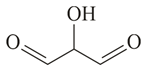

50% studentsanswered this correctly

Important Questions on Aldehydes, Ketones and Carboxylic Acids
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
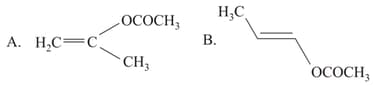
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
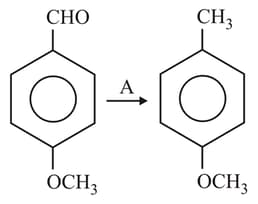
The reagent which can be used for the above conversion is
HARD
JEE Main
IMPORTANT
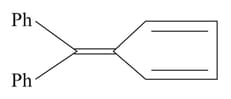
Which of the following will undergo base catalysed condensation reaction with cyclopentadiene to form this compound?
MEDIUM
JEE Main
IMPORTANT
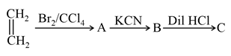
In the above reaction sequence, the product is
MEDIUM
JEE Main
IMPORTANT
Predict the compound in the following series of reactions.
HARD
JEE Main
IMPORTANT
