Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
The order of base catalysed hydrolysis of ester is
(1)
(2) 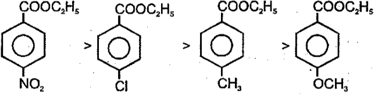
The reaction is sterically as well as electronically controlled reaction.
The order of base catalysed hydrolysis of ester is


Important Questions on Aldehydes, Ketones and Carboxylic Acids
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Acid catalysed hydrolysis of ester is reversible while base catalysed hydrolysis is irreversible.
In acid catalysèd ester hydrolysis carboxylic acid is formed on which nucleophilic attack of alcohol is possible but in base catalysed ester hydrolysis carboxylate anion is formed on which nucleophilic attack is not possible.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Acetate ion is more basic than the methoxide ion.
The methoxide ion is resonance stabilized.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Hydroxybenzoic acid has a lower boiling point than hydroxybenzoic acid.
Hydroxybenzoic acid has intermolecular hydrogen bonding.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Alkyl isocyanides in acidified water give alkyl formamides.
In isocyanides, carbon first acts as a nucleophile and then as an electrophile.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
butenal lacks enolizable atom, to carbonyl group still, it has sufficient acidic character.
The conjugate base of butenal is stabilised by resonance.
Each of these questions contains an Assertion followed by reason. Read them carefully and answer the question on the basis of following options. You have to select the one that best describes the two statements.
Dimethylpropanal undergoes Cannizzaro reaction with conc. .
Cannizzaro reaction is a disproportionation reaction.
Directions: Each of these questions contain an assertion followed by reason. Read them carefully and answer the question on the basis of the following options. You have to select the one that best describes the two statements.
Assertion: Alkyl isocyanides in acidified water give alkyl formamides.
Reason: In isocyanides, carbon first acts as a nucleophile and then acts as an electrophile.
