EASY
Earn 100
Electron gain enthalpy is the tendency of an isolated gaseous atom while electronegativity is the tendency to attract the shared pair of electrons in .
50% studentsanswered this correctly
Important Questions on Classification of Elements and Periodicity in Properties
EASY
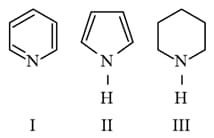
Arrange the following amines in the decreasing order of basicity.
HARD
Which of the following elements have the similar value of electronegativity?
HARD
Among alkali metals, which element do you expect to be least electronegative and why?
HARD
The ionisation potential and electron affinity of fluorine are and respectively. Calculate the electronegativity of fluorine on the Mulliken scale and the Pauling scale:
MEDIUM
Correct order of Pauling's electronegativity
HARD
Select the correct statement(s).
EASY
What is the difference between electronegativity and electron gain enthalpy?
MEDIUM
Ionisation energies of atoms A and B are and k cal mol-1 respectively. The electron affinities of these atoms are and k cal mol-1 respectively. Then which is the correct statement regarding electronegativity
EASY
If the ionization enthalpy and electron gain enthalpy of an element are 275 and 86 kcal mol-1 respectively, then the electronegativity of the element on the Pauling scale is:
HARD
Covalent radius of is . Calculate its electronegativity at the Alfred-Rochow scale:
HARD
Calculate the electronegativity of carbon at Pauling's scale?
Given:
Electronegativity of hydrogen
MEDIUM
Correct order of electronegativity of and on Pauling scale is:
MEDIUM
Calculate the electronegativity of fluorine using the following data.
,
,
and the electronegativity of .
EASY
If the ionization enthalpy and electron gain enthalpy of an element are 275 and 86 kcal respectively, then the electronegativity of the element on the Pauling scale is
MEDIUM
The formation of the oxide ion, requires first an exothermic and then an endothermic step as shown below :
This is because
MEDIUM
If and , the Pauling electronegativity of is about:
(Given: electronegativity of ).
MEDIUM
If the ionization enthalpy and electron gain enthalpy of an element are and , respectively, then the electronegativity of the element based on the Pauling scale is:
EASY
Cotyledons are also called-
EASY
The correct option with respect to the Pauling electronegativity values of the elements is:
EASY
Which of the following relation is correct

