HARD
Earn 100
Figure shows a horizontal cylindrical container of length 30 cm, which is partitioned by a tight-fitting separator. The separator is diathermic but conducts heat very slowly. Initially the separator is in the state shown in the figure. The temperature of left part of cylinder is 100 K and that on right part is 400 K. Initially the separator is in equilibrium. As heat is conducted from right to left part, separator displaces to the right. Find the displacement of separator after a long when gases on the two parts of cylinder are in thermal equilibrium.
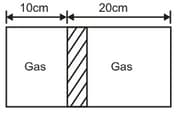
(a)10 cm
(b)20 cm
(c)30 cm
(d)40 cm
50% studentsanswered this correctly
Important Questions on Kinetic Theory
MEDIUM
An ideal gas undergoes a circular cycle centred at , as shown in the diagram.
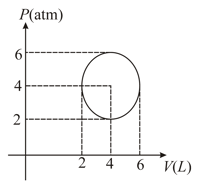
The maximum temperature attained in this process is close to
HARD
A long cylindrical pipe of radius is closed at its upper end and has an airtight piston of negligible mass as shown. When mass is attached to the other end of piston, it moves down by a distance, before coming to equilibrium. Assuming air to be an ideal gas, (see figure) is close to , one atmospheric pressure is ),
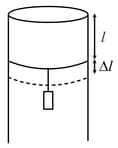
MEDIUM
EASY
EASY
EASY
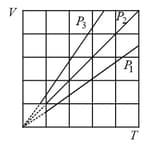
EASY
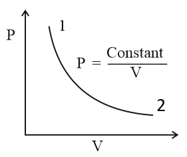
Out of the following which one correctly represents the diagram?
MEDIUM
MEDIUM
HARD
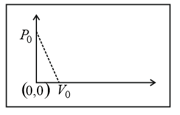
HARD
(Atmospheric pressure = of Hg)
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
( is universal gas constant and is the acceleration due to gravity)

