MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Identify the titration curve for equimolar mixture of and titrated with .
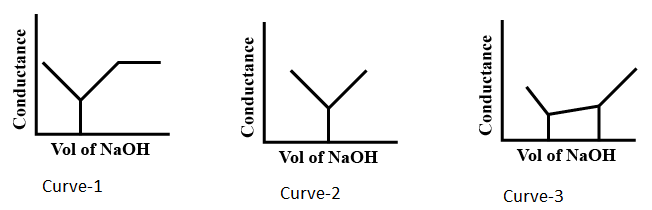
If your answer is first curve, write the answer as Curve-1.

50% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
for is for is:
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
