HARD
JEE Advanced
IMPORTANT
Earn 100
In the diagram shown in the figure, choose the correct options for the process .
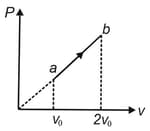

(a)Density of gas has reduced to half.
(b)Temperature of gas has increased to two times.
(c)Internal energy of gas has increased to four times.
(d) graph is a parabola passing through origin.
12.5% studentsanswered this correctly

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
EASY
JEE Advanced
IMPORTANT
Choose the wrong options
MEDIUM
JEE Advanced
IMPORTANT
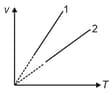
Along the line-, mass of gas is and pressure is . Along the line- mass of the same gas is and pressure is . Choose the correct options
EASY
JEE Advanced
IMPORTANT
Choose the correct options.
HARD
JEE Advanced
IMPORTANT
Show that the volume thermal expansion coefficient for an ideal gas at constant pressure is .
HARD
JEE Advanced
IMPORTANT
The volume of a diatomic gas is increased two times in a polytropic process with molar heat capacity, . How many times will the rate of collision of molecules against the wall of the vessel be reduced as a result of this process?
HARD
JEE Advanced
IMPORTANT
A perfectly conducting vessel of volume, contains an ideal gas at constant temperature, . A portion of the gas is let out and the pressure of the gas falls by, . (Density of the gas at is ). Find the mass of the gas which escapes from the vessel.
HARD
JEE Advanced
IMPORTANT
A thin-walled cylinder of mass, height, and cross-sectional area, is filled with a gas and floats on the surface of water. As a result of leakage from the lower part of the cylinder, the depth of its submergence has increased by . Find the initial pressure, of the gas in the cylinder if the atmospheric pressure is, and the temperature remains constant.
MEDIUM
JEE Advanced
IMPORTANT
Find the minimum attainable pressure of an ideal gas in the process ,, where, and are positive constants and is the volume of one mole of gas.