HARD
11th CBSE
IMPORTANT
Earn 100
crystallises in the same type of lattice as does . Given that and . Calculate the ratio of the side of the unit cell of to that of .
(a)
(b)
(c)
(d)
(e)
50% studentsanswered this correctly

Important Questions on States of Matter: Solid State
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
MEDIUM
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
HARD
11th CBSE
IMPORTANT
A metal has cubic close-packed () arrangement, the layer sequence of which is shown below:
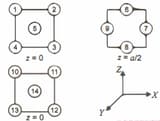
A face diagonal passes through the centre of atoms and the centre (s) of which other atoms?
HARD
11th CBSE
IMPORTANT
