The atmospheric density at an altitude is about molecules per .
Assuming the molecular diameter to be , find the mean free path.
Explain whether the predicted value is meaningful.
Given:- Boltzmann constant()=.

Important Questions on The Kinetic Theory of Gases
In a certain particle accelerator, protons travel around a circular path of diameter in an evacuated chamber, whose residual gas is at and pressure.
(a) Calculate the number of gas molecules per cubic centimeter at this pressure.
(b) What is the mean free path of the gas molecules if the molecular diameter is ?
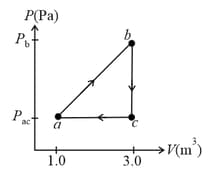
A sample of an ideal gas is taken through the cyclic process as shown.
The scale of the vertical axis is set by and .
At point , .
How many moles of gas are in the sample?
What are the temperature of the gas at point ?
temperature of the gas at point ?
total work done by the gas during the cycle?
net energy absorbed by the gas as heat during the cycle?
Assume of an ideal gas is taken from a volume of to a volume of via an isothermal expansion at .
(a) How much energy is transferred as heat during the process?
(b) Is the transfer to or from the gas?
A hydrogen molecule (diameter ) travelling at the speed, escapes from a furnace into a chamber containing cold argon atoms (diameter ) at a density atoms per .
What is the speed of the hydrogen molecule?
If it collides with an argon atom, what is the closest distance, their centers can be, considering each as spherical?
What is the initial number of collisions per second experienced by the hydrogen molecule? (Assume the argon atoms to be stationary)
