Which one is not correct for gaseous state obeying Van der Waal's equation?
Important Questions on States of Matter
[Given : " a " and " b " are standard parameters for van der Waals' gas]
Given below are two statement : one is labelled as Assertion and the other is labelled as Reason .
Assertion is adsorbed to a large extent than on activated charcoal.
Reason : has a higher critical temperature than
In the light of the above statements, choose the most appropriate answer from the options given below.
The number of statement's, which are correct with respect to the compression of carbon dioxide from point (a) in the Andrews isotherm from the following is _________.
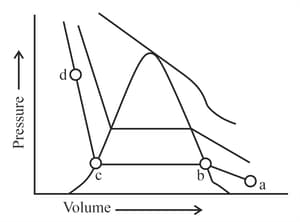
A. Carbon dioxide remains as a gas upto point (b)
B. Liquid carbon dioxide appears at point (c)
C. Liquid and gaseous carbon dioxide coexist between points (b) and (c)
D. As the volume decreases from (b) to (c), the amount of liquid decreases
A gas has a compressibility factor of and a molar volume of at a temperature of and pressure . If it shows ideal gas behaviour at the same temperature and pressure, the molar volume will be . The value of is _________
[Use: Gas constant, ]
Among the following gases, the order of liquefiability is
a)
b)
c)
d)
The isotherms of a gas are shown below :
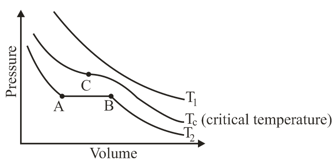
Among the following,
(i) At , the gas cannot be liquefied
(ii) At point , liquid starts to appear at
(ii) is the highest temperature at which the gas can be liquefied
(iv) At point , a small increase in pressure condenses the whole system to a liquid
The correct statements are :
Which of the following gases can be absorbed in more proportion?
| Gas | ||||
|---|---|---|---|---|
Which gas is expected to have the highest critical temperature?

