MEDIUM
Earn 100
Write Kelvin Planck's statements of the second law of thermodynamics.
Important Questions on Thermodynamics
MEDIUM
Which of the following relations is correct?
EASY
MEDIUM
EASY
EASY
HARD
EASY
HARD
MEDIUM
EASY
When steam condenses to water at , the entropy of the system decreases. What must be true if the second law of thermodynamics is to be satisfied?
[Hint: For a spontaneous process must be positive.]
HARD
The entropy change for the reaction,
is :
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
HARD
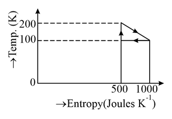
The efficiency of the reversible cycle in the given figure is