MEDIUM
Earn 100
Write a chemical equation for the action of ammonium hydroxide on ions.
Important Questions on Analytical Chemistry
HARD
The salt solution which does not react with ammonium hydroxide is:
MEDIUM
MEDIUM
MEDIUM
MEDIUM
A chloride which forms a precipitate that is soluble in excess of ammonium hydroxide, is
EASY
MEDIUM
HARD
HARD
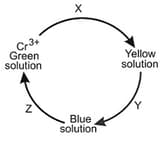
EASY
EASY
MEDIUM
Ammonium hydroxide is added to ferric chloride solution?
MEDIUM
MEDIUM
Copy and complete the following table:
| Compound | Solubility in sodium Hydroxide | Solubility in Ammonium Hydroxide |
| Copper hydroxide | ||
| Aluminium hydroxide | ||
| Zinc hydroxide |
HARD
EASY
MEDIUM
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
HARD
Read the following short write up and answer subsequent questions based.
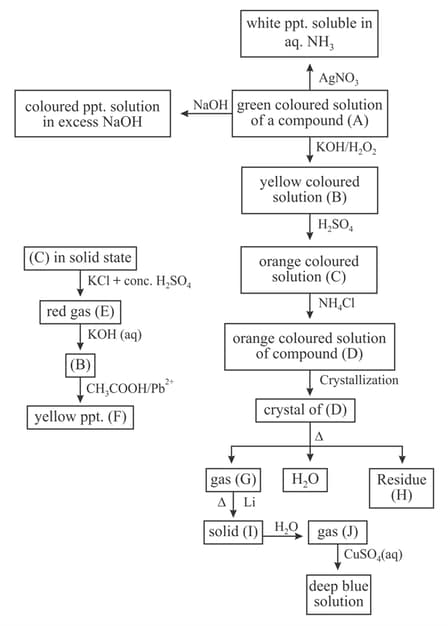
Compound A, B and C are respectively :

