MEDIUM
Earn 100
Zinc will react with dilute Sulphuric Acid to form salt and hydrogen gas
50% studentsanswered this correctly
Important Questions on Sorting Materials into Groups
EASY
EASY
EASY
EASY
MEDIUM
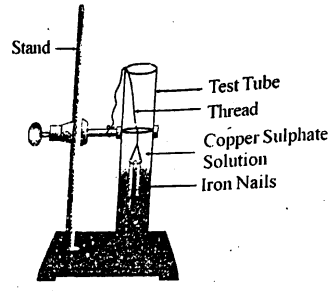
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
What is your observation in the following case:
Burning of magnesium in air.
MEDIUM
HARD
EASY
EASY
EASY
Complete the following reaction:
HARD
MEDIUM

