Molecular Orbital Theory
Important Questions on Molecular Orbital Theory
Amongst the following elements whose electronic configurations are given below, the one having the highest ionisation enthalpy is
Molecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital (BMO) and antibonding molecular orbital (ABMO). The energy of antibonding orbital is raised above the parent atomic orbitals that have combined and the energy of the bonding orbital is lowered than the parent atomic orbitals. Energies of various molecular orbitals for elements hydrogen to nitrogen increase in the order :
and for oxygen and fluorine order of energy of molecular orbitals is given below :
Different atomic orbitals of one atom combine with those atomic orbitals of the second atom which have comparable energies and proper orientation. Further, if the overlapping is head-on, the molecular orbital is called Sigma and if the overlap is lateral, the molecular orbital is called pi. The molecular orbitals are filled with electrons according to the same rules as followed for filling of atomic orbitals. However, the order for filling is not the same for all molecules or their ions. Bond order is one of the most important parameters to compare the strength of bonds.
In which of the following molecules, molecular orbital is filled after , and molecular orbitals?
Which of the following molecular orbitals has the maximum number of nodal planes?
Molecular orbitals are formed by the overlap of atomic orbitals. Two atomic orbitals combine to form two molecular orbitals called bonding molecular orbital (BMO) and antibonding molecular orbital (ABMO). The energy of antibonding orbital is raised above the parent atomic orbitals that have combined and the energy of the bonding orbital is lowered than the parent atomic orbitals. Energies of various molecular orbitals for elements hydrogen to nitrogen increase in the order :
and for oxygen and fluorine order of energy of molecular orbitals is given below :
Different atomic orbitals of one atom combine with those atomic orbitals of the second atom which have comparable energies and proper orientation. Further, if the overlapping is head-on, the molecular orbital is called Sigma and if the overlap is lateral, the molecular orbital is called pi. The molecular orbitals are filled with electrons according to the same rules as followed for filling of atomic orbitals. However, the order for filling is not the same for all molecules or their ions. Bond order is one of the most important parameters to compare the strength of bonds.
Which of the following statements is correct?
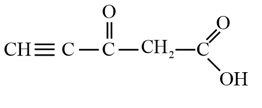
Predict the hybridisation of each carbon in the molecule of organic compound given below. Also indicate the total number of sigma and pi bonds in this molecule.
Use the molecular orbital energy level diagram to show that would be expected to have a triple bond, , a single bond and , no bond.
What is the effect of the following processes on the bond order in and ?
(i) (ii)
Explain the non linear shape of and non planar shape of using valence shell electron pair repulsion theory.
Diamagnetic species are those which contain no unpaired electrons. Which among the following are diamagnetic?
Which of the following statements are correct about ?
Which of the following options represents the correct bond order:
Which of the following statement is not correct from the view point of molecular orbital theory?
Which of the following order of energies of molecular orbitals of is correct?

