Van Der Waals Equation
Author:Embibe Experts
Chemistry
IMPORTANT
Important Questions on Van Der Waals Equation
MEDIUM
IMPORTANT
Which of the following is/are correct about critical conditions of real gas following Vander wall's equation of state :
HARD
IMPORTANT
Consider the given plots for four gases at . If the slope of line is , what is the relationship between Vander Waal's constants '' and ''? (Ignore the higher terms of Virial equation)
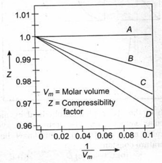
EASY
IMPORTANT
The critical constant for water are and $0.0566$ litre Calculate .

