Chemical Properties of Alkanes
Chemical Properties of Alkanes: Overview
This topic covers concepts, such as, Chemical Properties of Alkanes, Halogenation of Alkanes, Reaction of Alkanes with Steam & Controlled Oxidation of Alkanes etc.
Important Questions on Chemical Properties of Alkanes
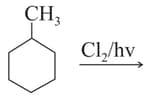
How many chiral compounds are possible on mono chlorination of 2-methyl butane?
Only two isomeric monochloro derivatives are possible for
Which of the following is the chemical reaction of methane with steam?
Write the chemical reaction of methane with steam?
What happens when methane reacts with steam?
The correct option referenced to the below statements is
Statement (i) : In the combustion of methane, water is one of the products.
Statement (ii): Combustion of of methane gives of water.
Consider the following reaction,
The reaction is named as
Which of the following metals do not react with steam?
What are formed when a metal reacts with steam?
Number of - bonds and -bonds present in 1,3,5,7-Octatetra-ene are ?
When n-heptane is passed over catalyst at 773K the product formed is?
A compound reacts with in presence of sunlight forming only one mono-chlorinated product, but it does not react with in dark. What is the correct option?
How many number of mole(s) of oxygen is/are required in the complete combustion of ?
Which of the statements is/are true about the reactivity of halogenation of alkanes? The reactivity order is
I. Lower the activation energy for the chain initiation step, more reactive is the halogen.
II. Lower the activation energy for the first chain propagation step, more reactive is the halogen
III. More negative is the overall heat of the reaction of halogenation of alkane, more reactive is the halogen
IV. Lower the activation energy for the second chain propogation step, more reactive is the halogen.
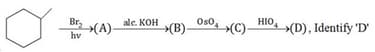
During monochlorination of ethylcyclopropane, Total number of theoretically products (including stereo) are
Calculate the % of major monochlorinated product obtained by chlorination of 2, 2, 5 trimethylhexane. Relative rate of alkyl radical formation by a chlorine radical at room temperature is 5: 4 : 1 (3° : 2° : 1°).
At which carbon chlorination would be yielding highest?
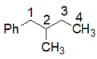
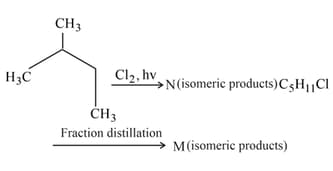
Find the total number of isomeric products in the following reaction.