Electronic Effects
Electronic Effects: Overview
This topic covers concepts, such as, Electron Displacement Effects in Covalent Bonds: Electronic Effects, Localised Electrons, Order of Groups Exhibiting Negative Inductive Effect & Cross-conjugated System etc.
Important Questions on Electronic Effects
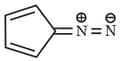
The most stable canonical structure of this molecule is:
In which of the following molecules / ions resonance structures are equivalent:
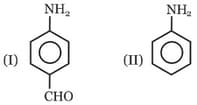
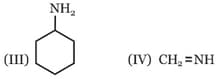
Among these compounds, the correct order of C–N bond length is :
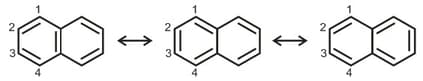
There are three canonical structures of naphthalene. Examine them and find the correct statement among the following:
In which of the following pairs, is the first species is more stable than second?
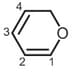
In this molecules, π-electron-density is more on
In which of the following molecules π-electron density in ring is maximum ?
In which of the following molecules π-electron density in ring is minimum ?
Which of the following compounds has maximum electron density in ring ?
Which of the following compounds has maximum electron density in ring ?
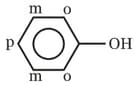
In phenol, π - electron - density is maximum on
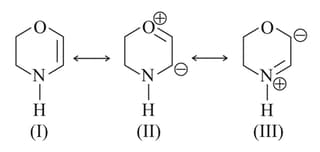
The least stable canonical structure among these is
The order of stability of these canonical forms is:
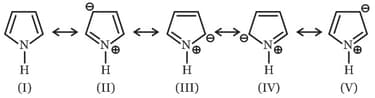
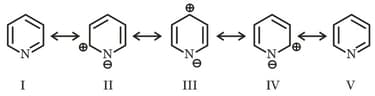
Among these canonical structures of pyridine, the correct order of stability is
Among these three canonical structures (through more are possible) what would be their relative contribution in the hybrid?
is more stable than because
Among these canonical structures which one is least stable ?
Among, these, which are canonical structures?
In which of the following molecules - group is not coplanar with phenyl ring?
In which of the following molecules resonance takes place through out the entire system
