First Law of Thermodynamics
First Law of Thermodynamics: Overview
This topic covers concepts such as Sign Convention in Thermodynamics, First Law of Thermodynamics, First Law of Thermodynamics in Isochoric Process, Heat in Isochoric Process, and First Law of Thermodynamics in Isobaric Process.
Important Questions on First Law of Thermodynamics
An ideal gas is allowed to expand freely against vacuum in a rigid insulated container. The gas undergoes
When heat is supplied to the system then is ____ and is ____ when heat is extracted from the system. Choose the appropriate option.
The ratio of work done by an ideal monoatomic gas to the heat supplied to it in an isobaric process is
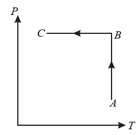
The diagram of a system undergoing thermodynamic transformation is shown in figure. The work done by the system in going from is and heat is given to the system. The change in internal energy between and is
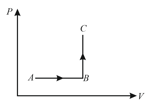
A monatomic ideal gas undergoes a process . The heat given to the gas is,
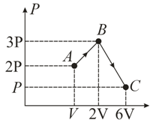
One mole of an ideal monatomic gas is taken through a semicircular thermodynamic process shown in the diagram. The heat supplied to the system is
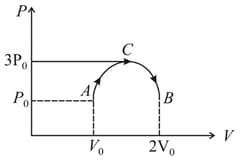
The workdone on the system in changing the state of a gas adiabatically from equilibrium state to equilibrium state is . If the gas is taken from state to through another process in which the net heat absorbed by the system is , then the net workdone by the system in the later case is
An electric heater supplies heat to a system at a rate of If the system performs Work at a rate of joules per second, the rate at which internal energy changes is
Two cylinders and of equal capacity are connected to each other via a stopcock. A contains an ideal gas at standard temperature and pressure. is empty. Then the entire system is thermally insulated. The stopcock is suddenly opened. After the gas reaches equilibrium the temperature of the gas measured. Which of the following is correct?
of water at , is completely converted to steam at , occupies . The increase in the internal energy of the molecules is:
(take the pressure , Latent heat of vaporisation )
As shown in the figure, the amount of heat absorbed along the path is and the amount of work done by the system is . If the amount of work done along the path is the amount of heat absorbed will be:
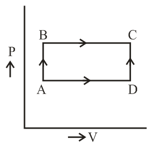
One mole of a perfect gas in a cylinder fitted with a piston has a pressure , volume and temperature . If the temperature is increased by keeping pressure constant, the increase in volume is :-
For an adiabatic compression, the quantity
Select the correct statment for an ideal gas under going following process.
An ideal gas is subjected to cyclic process involving four thermodynamic states. The amounts of heat and work involved in each of these states are
The ratio of the net work done by the gas to the total heat absorbed by the gas is . The values of and respectively are
Which one of the following is not true in a thermodynamic process?
When the state of a gas adiabatically changed from an equilibrium state to another equilibrium state an amount of work done on the system is . If the gas is taken from state to via a process in which the net heat absorbed by the system is , the word done by the substance will be
An electric heater supplies heat to a system at a rate of . If system performs work at a rate of , the rate of increase in internal energy is
One mole of an ideal monoatomic gas undergoes a cyclic process, as shown in the figure. If the temperature of the gas at state is and at state is , then heat exchanged during process , is
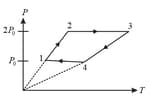
moles of an ideal monoatomic gas is carried from a state to state along a straight line path in a diagram. The amount of heat absorbed by gas in the process is given by
