Valence Bond Theory
Valence Bond Theory: Overview
This Topic covers sub-topics such as Valence Bond Theory, Limitations of Valence Bond Theory, Explanation of Valence Bond Theory, Magnetic Properties of Coordination Compounds and, Bonding in Coordination Compounds
Important Questions on Valence Bond Theory
The spin only magnetic moment of the complex in BM is
In 20th century, German scientist Werner succeeded in clarifying the structures of the five compounds consisting of platinum, chlorine, and ammonia. Some of the properties of these compounds are shown below in the table.
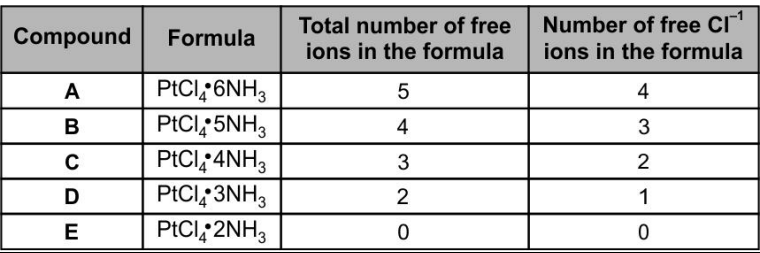
Predict the shape of each compound.
The structure of which of the following chloro species can be explained on the basis of hybridisation?
Which of the following compound has tetrahedral geometry?
Which of the following is an outer orbital complex?
Amongst the following ions which one has the highest paramagnetism ?
Write an example for a linear complex ion with coordination number two.
What is the hybridisation of central metal atom of complex compound with coordination number .
The metal with coordination number two in complex compound forms _____ complex.
How valence bond limits the electroneutrality and back bonding?
What are the drawbacks of valence bond theory in coordination compounds?
What happens if reacts with ?
Explain that is a low spin complex while is a high spin complex.
Explain is an inner orbital complex whereas is an outer orbital complex.
Which of the following compounds has coordination number as 5?
Explain about the structure of the complex .
Explain the case giving appropriate reasons : Nickel does not form low spin octahedral complexes.
