Academic Experts Solutions for Chapter: Chemical Kinetics & Nuclear Chemistry, Exercise 1: Exercise
Academic Experts Chemistry Solutions for Exercise - Academic Experts Solutions for Chapter: Chemical Kinetics & Nuclear Chemistry, Exercise 1: Exercise
Attempt the free practice questions on Chapter 2: Chemical Kinetics & Nuclear Chemistry, Exercise 1: Exercise with hints and solutions to strengthen your understanding. Practice Book for KVPY Aptitude Test - Stream SX Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Academic Experts Solutions for Chapter: Chemical Kinetics & Nuclear Chemistry, Exercise 1: Exercise with Hints & Solutions
For an elementary reaction of reversible nature, net rate is: , hence, given reaction is:
A substance '' decomposes in solution following the first order kinetics. Flask contains of solution of and flask contains of solution. After , the concentration of in flask becomes . What will be time for concentration of in flask to become ?
The rate constant for the reaction, , is . If the rate is , then determine the concentration of in .
In the biologically-catalysed oxidation of ethanol, the concentration of ethanol decreases in a first order reaction from to in . Determine the rate constant of the reaction. If answer is then x is.
Under the same reaction conditions, initial concentration of of a substance becomes half in and through first order and zero order kinetics, respectively. Determine the ratio of the rate constant for first order and zero order of the reaction.
For a first order reaction , the temperature dependent rate constant was found to follow the equation . The activation energy is _____.
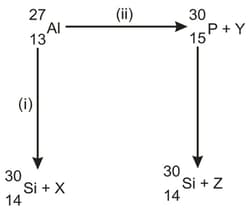
Bombardment of aluminum by -particle leads to its artificial disintegration in two ways, and as shown. Determine the products and .
is radioactive and it emits and particles to form . Calculate the number of and particles emitted in this conversion. An ore of is found to contain and in the weight ratio of , the half life period of is years. Calculate the age of the ore. If answer is then y is.
