Academic Experts Solutions for Chapter: Solution & Colligative Properties, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS)
Academic Experts Chemistry Solutions for Exercise - Academic Experts Solutions for Chapter: Solution & Colligative Properties, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS)
Attempt the free practice questions on Chapter 3: Solution & Colligative Properties, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS) with hints and solutions to strengthen your understanding. Practice Book for KVPY Aptitude Test - Stream SX Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Academic Experts Solutions for Chapter: Solution & Colligative Properties, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS) with Hints & Solutions
A solution containing of nicotine in of water freezes degrees below the normal freezing point of water. If the molal freezing point depression constant, , then the molar mass of nicotine is:
At , the ratio of osmotic pressures of two solutions of a substance with concentrations of and , respectively, is:
At the vapour pressure of two pure liquids, and are and , respectively. If in a mixture of and , the vapour pressure is , the mole fractions of in the liquid and in the vapour phase, respectively, are
The variation of solubility of four different gases ( etc.,) in a given solvent with pressure at a constant temperature is shown in the plot.
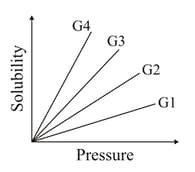
The gas with the highest value of Henry's law constant is
In of water, of a non-volatile compound is dissolved. At the vapour pressure of the solution is . Assuming that the compound does not undergo association or dissociation, the molar mass of the compound in is
is precipitated when is added to a solution of . If the final concentration of is , the concentration of in the solution is
[Solubility product for ]
A piece of metal weighing is heated to and dropped into of cold water in an insulated container at . If the final temperature of the water in the container is , the specific heat of the metal in . is
The plot of total vapour pressure as a function of mole fraction of the components of an ideal solution formed by mixing liquids and is:
