Aidan Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: Elements, Compounds and Mixtures, Exercise 2: Exercise 2
Aidan Gill Science Solutions for Exercise - Aidan Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: Elements, Compounds and Mixtures, Exercise 2: Exercise 2
Attempt the free practice questions on Chapter 4: Elements, Compounds and Mixtures, Exercise 2: Exercise 2 with hints and solutions to strengthen your understanding. Cambridge Lower Secondary Science Stage 8 : Workbook solutions are prepared by Experienced Embibe Experts.
Questions from Aidan Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: Elements, Compounds and Mixtures, Exercise 2: Exercise 2 with Hints & Solutions
Diffusion will occur if there is a difference in:
Tick one box.
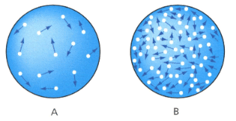
The two particle diagrams shows the same gas at different pressures.
State which diagram shows the gas at higher pressure _____.
Which statements are true?
Tick two boxes.
Look at the diagram. It shows a sealed gas syringe.
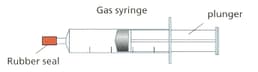
Describe what will happen to the pressure of the gas as the plunger is pushed in.
Look at the diagram. It shows a sealed gas syringe.
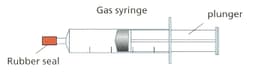
Use your knowledge of particle theory to explain the answer.what will happen to the pressure of the gas as the plunger is pushed in.
Pierre was about to go out when he notices that his bicycle has a flat tyre.
He is looking worried because he does not know how to repair it.
Describe what happened to the amount of air in the tyre. Suggest a reason for your answer.
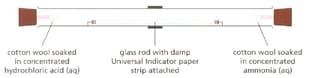
When concentrated hydrochloric acid reacts with concentrated Ammonia solution a white compound is formed.
A teacher set up of the following demonstration.
Why was universal indicator included in the demonstration?
When concentrated hydrochloric acid reacts with concentrated Ammonia solution a white compound is formed.
A teacher set up of the following demonstration.
Explain this observation.