Aiden Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: Preparing Salts, Exercise 1: Exercise 1
Aiden Gill Science Solutions for Exercise - Aiden Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: Preparing Salts, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 6: Preparing Salts, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Cambridge Lower Secondary Science Stage 9: Workbook solutions are prepared by Experienced Embibe Experts.
Questions from Aiden Gill, Heidi Foxford and, Dorothy Warren Solutions for Chapter: Preparing Salts, Exercise 1: Exercise 1 with Hints & Solutions
The formula of calcium sulphate is . What is the total number of atoms present?
Name the salt when magnesium reacts with hydrochloric acid.
The diagram shows some sulphuric acid in measuring cylinder.
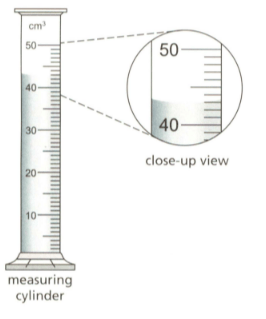
How much sulphuric acid is in the measuring cylinder?
(A) (B) (C)
Enter your correct answer as A, B or C.

Describe the safety precautions you should take when making a salt using calcium metal.
Keep it away from naked flames because one of the substances involved is _____.
Wear _____ to protect your eyes from splashes, which are _____.
Do not touch calcium with your hands -wear _____.
Dilute hydrochloric acid is classified as 'low hazard'. Describe a safety precaution you should always take when using dilute acids.
Explain why some salts have large crystals, while others have small crystals.
