Stereochemical Aspects of Nucleophilic Substitution Reactions of Haloalkanes and Haloarenes
Important Questions on Stereochemical Aspects of Nucleophilic Substitution Reactions of Haloalkanes and Haloarenes
Chiral molecules are :
The enantiomeric excess and observed rotation of a mixture containing of and of -butanol are respectively (If the specific rotation of enantiomerically pure butanol is unit).
Which of the following pairs of compounds are enantiomers:
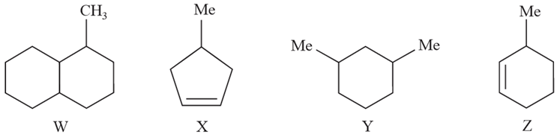
How many chiral centres are present in the following compounds?
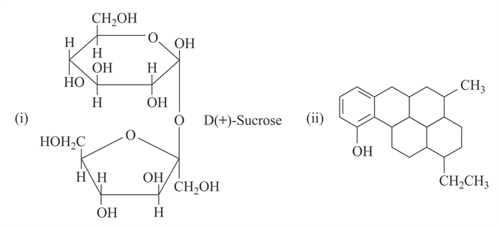
The number of chiral carbon atoms in the compound and respectively would be
Indicate whether each of the following compound is '' or ''.

Which configuration will be adopted by the product at carbon atoms marked () and (), respectively, in the given reaction?
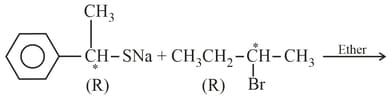
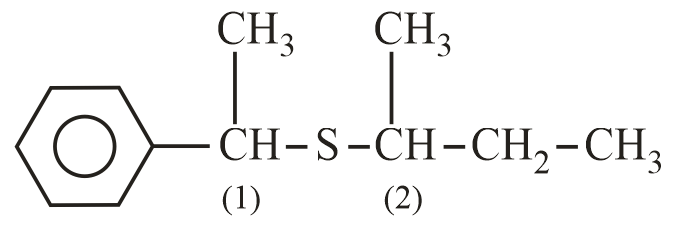
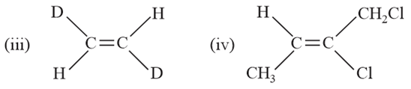
Cyclohexene reacts with limited amount of bromine in the presence of light to form product The correct statement about is
The total number of optically active compounds formed in the following reaction is
An enantiomerically pure acid is treated with racemic mixture of an alcohol having one chiral carbon. The ester formed will be
Racemic mixture is formed by mixing two

