Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 2: Exercise - 2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 2: Exercise - 2
Attempt the free practice questions on Chapter 33: Atomic Physics, Exercise 2: Exercise - 2 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Atomic Physics, Exercise 2: Exercise - 2 with Hints & Solutions
For the structural analysis of crystals, rays are used because:
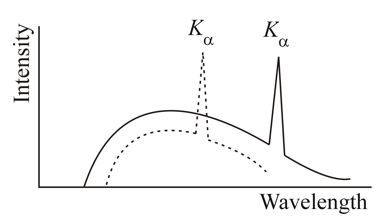
The figure shows the intensity-wavelength relations of rays coming from two different Coolidge tubes. The solid curve represents the relation for the tube in which the potential difference between the target and the filament is and the atomic number of the target material is . These quantities are and for the other tube. Then,
If is minimum wavelength produced in tube and is the wavelength of line. As the operating tube voltage is increased.
According to Moseley’s law the ratio of the slopes of the graph between and for and is :
If the frequency of emitted from the element with atomic number is , then the frequency of emitted from the element with atomic number would be (assume that screening constant for is ) :
An particle with a kinetic energy of makes a head on collision with a hydrogen in ground state atom moving towards it with a kinetic energy of The collision.
In an tube the electrons are expected to strike the target with a velocity that is of the velocity of light. The applied voltage should be
In and atom an electron excites to the fourth orbit. When it jumps back to the energy levels a spectrum is formed. Total number of spectral lines in this spectrum would be
