Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Exercise - 2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Exercise - 2
Attempt the free practice questions on Chapter 17: Thermodynamics, Exercise 2: Exercise - 2 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 2: Exercise - 2 with Hints & Solutions
When two nonreactive samples at different temperatures are mixed in an isolated container of negligible heat capacity the final temperature of the mixture can be
For an ideal gas,
A cyclic process is shown in the P–V diagram. ( and are isothermal)
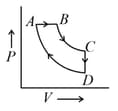
Which of the following curves represent the same process?
A cyclic process of an ideal monoatomic gas is shown in figure. The correct statement is (are)
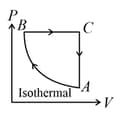
A gas kept in a container, if the container is of finite conductivity, then the process
Oxygen, nitrogen and helium gas are kept in three identical adiabatic containersand , respectively at equal pressure. When the gases are pushed to half their original volumes. (initial temperature is same)
Which of the following statement/s in case of a thermodynamic process is /are correct ?
If a system is made to undergo a change from an initial state to a final state by adiabatic process only, then
