Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise-3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise-3
Attempt the practice questions on Chapter 18: Kinetic Theory of Gases, Exercise 3: Exercise-3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Medical: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise-3 with Hints & Solutions
One mole of an ideal gas goes from an initial state to final state via two processes. It first undergoes isothermal expansion from volume to and then its volume is reduced from to at constant pressure. The correct diagram representing the two processes is:
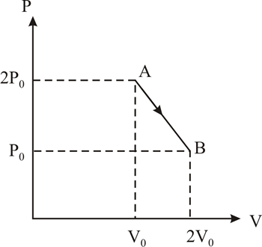
In the given diagram, what is the relation between pressure and ?

One of a diatomic gas is at pressure of . The density of the gas is . What is the energy of the gas due to thermal motions?
A container with insulating walls is divided into two equal parts by a partition fitted with a valve. One part is filled with an ideal gas at a pressure and temperature , whereas the other part is completely evacuated. If the valve is suddenly opened, the pressure and temperature of the gas will be
moles of an ideal gas undergoes a process as shown in the figure. The maximum temperature of the gas during the process will be:
An ideal gas is enclosed in a cylinder at pressure of and temperature, . Then mean time between two successive collisions is . If the pressure is doubled and temperature is increased to , the mean time between two successive collisions will be close to
A vertical closed cylinder is separated into two parts by a frictionless piston of mass and of negligible thickness. The piston is free to move along the length of the cylinder. The length of the cylinder above the piston is and that below the piston is such that . Each part of the cylinder contains moles of an ideal gas at equal temperature . If the piston is stationary, its mass, , will be given by ( is the universal gas constant and is the acceleration due to gravity)
A flask containing air at is corked up at atmospheric pressure. The cork can be forced out by the pressure of atmosphere. To what temperature the flask should be heated to do that?
