Mass Defect and Binding Energy
Important Questions on Mass Defect and Binding Energy
The above is a plot of binding energy per nucleon , against the nuclear mass correspond to different nuclei. Consider four reactions:
(i)
(ii) .
(iii) and
(iv) ,
where is the energy released. In which reactions positive?
undergoes a decay by emitted $\beta^{+}$ then write its complete equation Given the mass value of and Calculate the Q-value of reaction.
If , and denote the masses of the nucleus , proton and neutron respectively in units of and represents its binding energy in , then
Helium nuclei combine to form an oxygen nucleus. The energy released in the reaction is if and
The dynamic mass of electron having rest mass and moving with speed is
In a fission reaction, the average binding energy per nucleon of and is whereas that of is . The total energy liberated will be about
and represent the mass of neutron and proton, respectively. An element having mass and neutron, -protons, then the correct relation will be
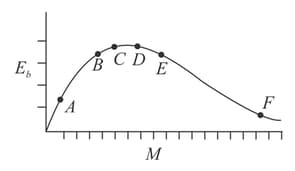
The binding energy per nucleon v/s mass number curve for nuclei is shown in the figure. and are four nuclei indicated on the curve. The process that would release energy is:
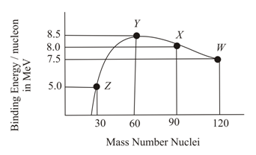
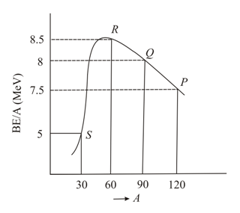
The figure shows a plot of binding energy per nucleon vs mass number for nuclei. Four nuclei, and are indicated on the curve. The process that would release energy is
and are masses of proton and neutron, respectively, at rest. If they combine to form a deuterium nucleus. The mass of the nucleus will be
Binding energy per nucleon is of the order of -
If is the mass of an oxygen isotope and are the masses of a proton and a neutron, respectively, the nuclear binding energy of the isotope is
If is the mass of an oxygen isotope , and are the masses of a proton and a neutron, respectively, the nuclear binding energy of the isotope is
The binding energies of two nuclei and and and joules. If then the energy released in the reaction , will be
Mass-energy equation was given by
amu is equivalent to
In the reaction if binding energies of and are respectively and (in ), then the energy released in this reaction is:
A fission reaction is given by where and are two particles. Considering to be at rest, the kinetic energies of the products are denoted by and respectively. Let the
binding energies per nucleon of and be and respectively. Considering different conservation laws, the correct option(s) is(are)
The masses of nucleus, neutrons and protons are and , respectively. If the nucleus has been divided into neutrons and protons, then,
For the stability of any nucleus,

