Anil Ahlawat Solutions for Chapter: Physical and Chemical Changes, Exercise 1: EXERCISES
Anil Ahlawat Science Solutions for Exercise - Anil Ahlawat Solutions for Chapter: Physical and Chemical Changes, Exercise 1: EXERCISES
Attempt the free practice questions on Chapter 5: Physical and Chemical Changes, Exercise 1: EXERCISES with hints and solutions to strengthen your understanding. NSO Science Olympiad Workbook Grade 7 solutions are prepared by Experienced Embibe Experts.
Questions from Anil Ahlawat Solutions for Chapter: Physical and Chemical Changes, Exercise 1: EXERCISES with Hints & Solutions
Observe the given reaction carefully and fill in the blanks by selecting an appropriate option.
Before the reaction, iron is (i) in colour and solution is (ii) in colour. After the reaction, iron gets (iii) deposits and the solution becomes (iv) in colour.
| (i) | (ii) | (iii) | (iv) | |
| (A) | Grey | Colourless | Red | Blue |
| (B) | Red | Blue | Grey | Green |
| (C) | Grey | Blue | Brown | Green |
| (D) | Red | Colourless | Grey | Green |
Observe the given experimental set-up carefully.
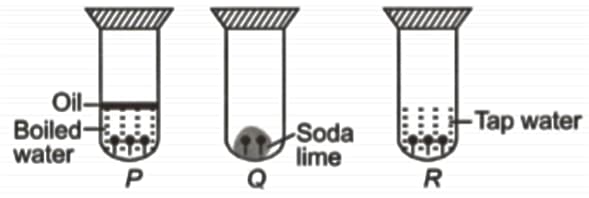
In which test tubes rusting of iron nails will not take place?
Kunal took few iron turnings and mixed them well with sulphur powder. He could separate the iron turnings with the help of a magnet. He heated the mixture for some time and tried to separate the iron turnings with the magnet, but he could not. Why?
Observe the given figure carefully and identify the substances marked as (p), (q) and (r).
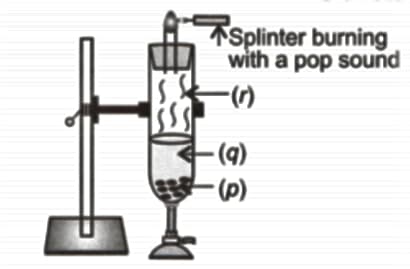
| (p) | (q) | (r) | |
| (A) | Zinc | Water | Carbon dioxide |
| (B) | Magnesium | Hydrochloric acid | Oxygen |
| (C) | Magnesium | Water | Carbon dioxide |
| (D) | Zinc | Hydrochloric acid | Hydrogen |
Study the given Venn diagram carefully.
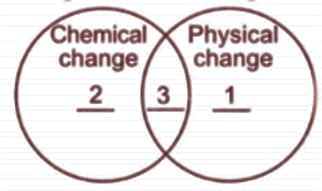
Identify , and .
| (A) | Melting of ice | Rusting of iron | Cutting a log of wood |
| (B) | Glowing of bulb | Bursting of tyre | Crystallisation |
| (C) | Stitching of garments | Burning a matchstick | Burning of a candle |
| (D) | Melting of ice | Decomposition of sugar | Sublimation |
Rupali classified a few changes occurring around us as shown in the table.
| S. No. | Change | Chemical | Physical | Reversible |
| 1. | Grinding of wheat | ✓ | x | x |
| 2. | Bursting of crackers | ✓ | x | x |
| 3. | Formation of clouds | x | ✓ | ✓ |
| 4. | Germination of seeds | ✓ | x | ✓ |
| 5. | Drying of wet cloths | x | ✓ | x |
Which of her observations are incorrect?
Read the given statements and select the correct option.
Statement : Many cut fruits, when kept in open turn brown.
Statement : Browning of fruits is due to a chemical reaction.
During the science activity, Ms Prabha demonstrated an experiment in the following steps:
Step : She burned a substance over the candle flame and white dazzling light was observed. Also, a white powdery ash was left behind.
Step : She mixed the white ash formed with a small amount of water. Then she dipped red and blue litmus papers into the solution one by one.
Which of the following observations are correct?
I. The substance burned in step was magnesium.
II. The white ash formed in step was magnesium oxide.
III. The solution formed in step was acidic in nature
IV. The solution formed in step turned blue litmus red.
