Anil Ahlawat Solutions for Chapter: Metals and Non-Metals, Exercise 1: EXERCISES
Anil Ahlawat Science Solutions for Exercise - Anil Ahlawat Solutions for Chapter: Metals and Non-Metals, Exercise 1: EXERCISES
Attempt the practice questions on Chapter 4: Metals and Non-Metals, Exercise 1: EXERCISES with hints and solutions to strengthen your understanding. NSO Science Olympiad Workbook Grade 8 solutions are prepared by Experienced Embibe Experts.
Questions from Anil Ahlawat Solutions for Chapter: Metals and Non-Metals, Exercise 1: EXERCISES with Hints & Solutions
Mr. Ramakant, a science teacher organised a quiz in the class. He stated few applications of non-metals and asked students to guess the names of the non-metals. Mark the correct option.
Read the given passage and fill in the blanks by selecting an appropriate option. Non-metals find wide applications in industries. For example i is used in electrodes, ii is used in welding metals, iii is used to make antiseptic creams while iv is used as a preservative due to its inert nature.
The oxide of sulphur (which can be further oxidised), when dissolved in water gives:
Study the given table carefully and select the appropriate option.
| Sample | Conductor of electricity | Malleability |
| I | x | |
| II | x | x |
| III | x | |
| IV |
Observe the given figure carefully.
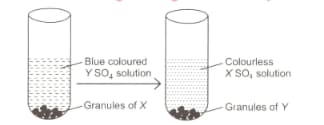
and respectively are
Aditya, a class student investigated the reactivity of four metals, iron, copper, zinc and an unknown metal . He arranged three experimental set-ups as shown in the diagram and observed the changes carefully.
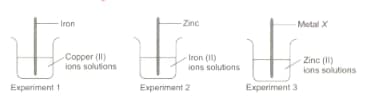
If reactions occur in all three beakers then the metal is
Each beaker contains two metal strips of same size fastened together and immersed in hydrochloric acid. After minutes, which beaker will contain the least amount of zinc ions?
Fill in the blanks with the most appropriate option.
'' is a very reactive metal, reacts vigorously with oxygen and water therefore, stored in .
'' is a non-metal, soft and dull and forms oxides with oxygen.
'' is very reactive , catches fire if exposed to air, therefore, stored in water.
'' does not react with dilute hydrochloric acid even on heating but it reacts with sulphuric acid. When it is exposed to moist air for long, it acquires a dull coating.
