Annie Termaat and Christopher Talbot Solutions for Chapter: How Can Our Energy Resources Be Accessed Fairly?, Exercise 18: ACTIVITY: Calculating enthalpy changes (ΔH) using bond energy tables
Annie Termaat Chemistry Solutions for Exercise - Annie Termaat and Christopher Talbot Solutions for Chapter: How Can Our Energy Resources Be Accessed Fairly?, Exercise 18: ACTIVITY: Calculating enthalpy changes (ΔH) using bond energy tables
Attempt the free practice questions on Chapter 10: How Can Our Energy Resources Be Accessed Fairly?, Exercise 18: ACTIVITY: Calculating enthalpy changes (ΔH) using bond energy tables with hints and solutions to strengthen your understanding. MYP By Concept 4&5 Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Annie Termaat and Christopher Talbot Solutions for Chapter: How Can Our Energy Resources Be Accessed Fairly?, Exercise 18: ACTIVITY: Calculating enthalpy changes (ΔH) using bond energy tables with Hints & Solutions
If in a chemical reaction, is positive, state the type of reaction involved.
If in a chemical reaction, is positive, outline the source of this thermal energy, in terms of bonds of the chemical reactants and products.
Calculate, using the information in the table, the enthalpy of combustion of ethanol.
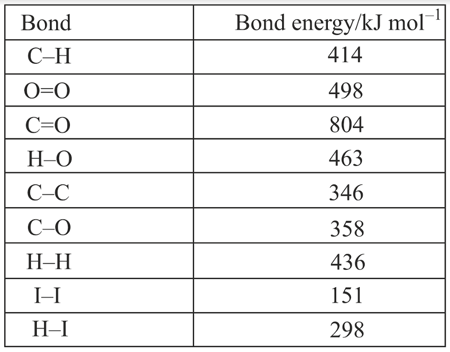
Calculate, using the information in the table, the enthalpy of combustion of octane , a major component of petrol.
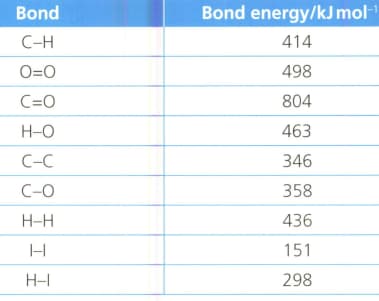
Calculate the energy released when mole of methane is burnt.
When of methane is burnt in oxygen, the heat evolved is . What is the heat of combustion (in ) of methane?
Calculate the energy released (heat produced) when of methane is burnt.
If a chemical reaction is positive: state the type of chemical reaction involved.
