Annie Termaat and Christopher Talbot Solutions for Chapter: How Do We Use Matter?, Exercise 2: ACTIVITY: Reflecting on the nature of glass
Annie Termaat Chemistry Solutions for Exercise - Annie Termaat and Christopher Talbot Solutions for Chapter: How Do We Use Matter?, Exercise 2: ACTIVITY: Reflecting on the nature of glass
Attempt the free practice questions on Chapter 2: How Do We Use Matter?, Exercise 2: ACTIVITY: Reflecting on the nature of glass with hints and solutions to strengthen your understanding. MYP By Concept 4&5 Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Annie Termaat and Christopher Talbot Solutions for Chapter: How Do We Use Matter?, Exercise 2: ACTIVITY: Reflecting on the nature of glass with Hints & Solutions
Describe the physical properties of glass? Why pure glass can be considered as super viscous liquid?
If kinetic molecular theory explains the movement and arrangement of particles from solid to liquid to gas as they heat or cool. Suggest how it explains the ductility of molten glass.
If kinetic molecular theory explains the movement and arrangement of particles from solid to liquid to gas as they heat or cool. How materials fuse together?
If kinetic molecular theory explains the movement and arrangement of particles from solid to liquid to gas as they heat or cool. Why a slowly cooled material might be stronger than one cooled quickly.
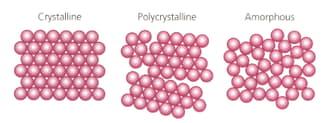
Which of these models for solids best describes the physical properties of glass?