B M Sharma Solutions for Chapter: Atomic Physics, Exercise 1: DPP
B M Sharma Physics Solutions for Exercise - B M Sharma Solutions for Chapter: Atomic Physics, Exercise 1: DPP
Attempt the free practice questions on Chapter 4: Atomic Physics, Exercise 1: DPP with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Optics and Modern Physics JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from B M Sharma Solutions for Chapter: Atomic Physics, Exercise 1: DPP with Hints & Solutions
The ratio of the speed of the electrons in the ground state of hydrogen to the speed of light in vacuum is
Minimum excitation potential of Bohr's first orbit in hydrogen atom is
Which of the following statements are true regarding Bohr's model of hydrogen atom?
Orbiting speed of electron decreases as it shifts to discrete orbits away from the nucleus.
Radii of allowed orbits of electron are proportional to the principal quantum number.
Frequency with which electrons orbit around the nucleus in discrete orbits is inversely proportional to the cube of principal quantum number.
Binding force with which the electron is bound to the nucleus increases as it shifts to outer orbits.
Select the correct answer using the codes given below
The de-Broglie wavelength of an electron in the first Bohr orbit is
In a hypothetical Bohr hydrogen, the mass of the electron is doubled. The energy, and the radius, of the first orbit, will be? ( is the Bohr radius)
Which of the following transitions in a hydrogen atom emits photon of the highest frequency?
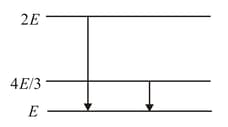
The following diagram indicates the energy levels of a certain atom when the system moves from level to , a photon of wavelength is emitted. The wavelength of photon produced during its transition from level to is
A double charged lithium atom is equivalent to hydrogen atom whose atomic number is . The wavelength of required radiation for emitting electron from first to third Bohr orbit in will be? (Ionisation energy of hydrogen atom is )
