B M Sharma Solutions for Chapter: Atomic Physics, Exercise 2: DPP
B M Sharma Physics Solutions for Exercise - B M Sharma Solutions for Chapter: Atomic Physics, Exercise 2: DPP
Attempt the free practice questions on Chapter 4: Atomic Physics, Exercise 2: DPP with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Optics and Modern Physics JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from B M Sharma Solutions for Chapter: Atomic Physics, Exercise 2: DPP with Hints & Solutions
The energy level diagram for a hydrogen-like atom is shown in the figure. The radius of its first Bohr orbit is

If the series limit of Lyman series for hydrogen atom is equal to the series limit of Balmer series for a hydrogen-like atom, then the atomic number of this hydrogen like atom will be
The following diagram indicates the energy levels of a certain atom when the system moves from level to . A photon of wavelength is emitted. The wavelength of photon produced during its transition from level to is . The ratio will be
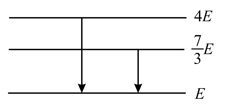
A hydrogen atom emits a photon corresponding to an electron transition from to . The recoil speed of hydrogen atom is almost (mass of proton )
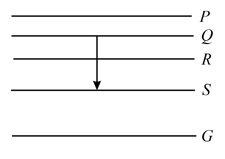
Energy levels , , of a certain atom corresponding to increasing values of energy, i.e., . If , and are the wavelengths of radiations corresponding to the transitions to , to and to respectively, which of the following statements is correct
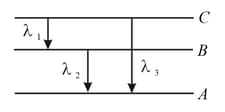
Figure shows the energy levels , , , and of an atom, where is the ground state. A red line in the emission spectrum of the atom can be obtained by an energy level change from to . A blue line can be obtained by the following energy level change
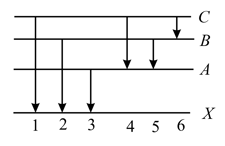
The figure indicates the energy level diagram of an atom and the origin of six spectral lines in emission (e.g. line no. arises from the transition from level to ). The following spectral lines will also occur in the absorption spectrum
A hydrogen like atom of atomic number is in an excited state of quantum number . It can emit a maximum energy photon of . If it makes a transition to quantum state , a photon of energy is emitted. The value of will be
