B M Sharma Solutions for Chapter: Kinetic Theory Of Gases, Exercise 2: DPP
B M Sharma Physics Solutions for Exercise - B M Sharma Solutions for Chapter: Kinetic Theory Of Gases, Exercise 2: DPP
Attempt the free practice questions on Chapter 3: Kinetic Theory Of Gases, Exercise 2: DPP with hints and solutions to strengthen your understanding. Chapterwise/Topicwise Daily Practice Problems (DPP) Waves & Thermodynamics JEE Main & Advanced solutions are prepared by Experienced Embibe Experts.
Questions from B M Sharma Solutions for Chapter: Kinetic Theory Of Gases, Exercise 2: DPP with Hints & Solutions
The initial temperature of a gas is . The gas is contained in a closed vessel. If the pressure of the gas is increased by . Calculate the increase in temperature of the gas.
A closed hollow insulated cylinder is filled with gas at and also contains an insulated piston of negligible weight and negligible thickness at the middle point. The gas on one side of the piston is heated to . If the piston moves , the length of the hollow cylinder is
The airtight and smooth pistons of a cylindrical vessel are connected with a string, as shown, initially, pressure and temperature of the gas are and . The atmospheric pressure is also . At a later time, the tension in the string is where is the cross-sectional area of the cylinder. At this time, the temperature of the gas has become

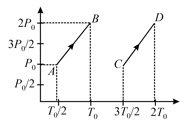
graph for the same number of moles of two ideal gases is shown in the figure. Find the path along which volume decreases.
A vessel of volume is separated into three equal parts by stationary semipermeable membranes. The left, middle and right parts are filled with of hydrogen, of oxygen and of nitrogen respectively. The left partition lets through the only hydrogen while the right partition lets through hydrogen and nitrogen. If the temperature in all is , the ratio of pressure in the three compartments will be?
A steel tank contains of ammonia gas at a pressure of and a temperature of . Later the temperature is and the pressure is .
Which of the following quantities is independent of the nature of the gas at same temperature?
Which of the following quantities depend on temperature only for a given ideal gas?
