B M Sharma Solutions for Chapter: Thermodynamics, Exercise 4: Archives
B M Sharma Physics Solutions for Exercise - B M Sharma Solutions for Chapter: Thermodynamics, Exercise 4: Archives
Attempt the free practice questions on Chapter 4: Thermodynamics, Exercise 4: Archives with hints and solutions to strengthen your understanding. PHYSICS FOR JOINT ENTRANCE EXAMINATION WAVES AND THERMODYNAMICS solutions are prepared by Experienced Embibe Experts.
Questions from B M Sharma Solutions for Chapter: Thermodynamics, Exercise 4: Archives with Hints & Solutions
Two moles of helium gas is taken over the cycle , as shown in the diagram.
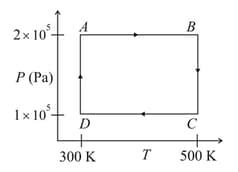
The work done on the gas in taking it from to is
Two moles of helium gas is taken over the cycle , as shown in the diagram.
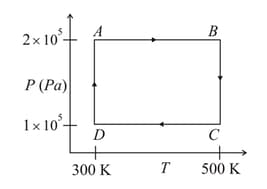
The net work done on the gas in the cycle is
A carnot engine operating between temperatures and has efficiency . When is lowered by , its efficiency increases to . Then and are, respectively
A carnot engine, whose efficiency is , takes in heat from a source maintained at a temperature of . It is desired to have an engine of efficiency . Then, the intake temperature for the same exhaust (sink) temperature must be
and are specific heats at constant pressure and constant volume, respectively. It is observed that for hydrogen gas, for nitrogen gas. The correct relation between and is
Two moles of ideal helium gas are in a rubber balloon at The balloon is fully expandable and can be assumed to require no energy in its expansion. The temperature of the gas in the balloon is slowly changed to The amount of heat required in raising the temperature is nearly
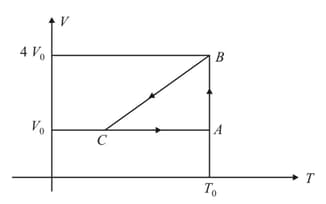
One mole of an ideal gas in initial state undergoes a cyclic process , as shown in the figure. Its pressure at is . Choose the correct option(s) from the following
One mole of a monatomic ideal gas undergoes four thermodynamic processes as shown schematically in the -diagram below. Among these four processes, one is isobaric, one is isochoric, one is isothermal and one is adiabatic. Match the processes mentioned in List-I with the corresponding statements in List-II.
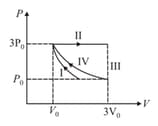
| List-I | List-II | ||
| P | In process I | 1. | Work done by the gas is zero |
| Q | In process II | 2. | Temperature of the gas remains unchanged |
| R | In process III | 3. | No heat is exchanged between the gas and its surroundings |
| S | In process IV | 4. | Work done by the gas is |
