Hydrogen Bonding and its Application
Important Questions on Hydrogen Bonding and its Application
The bond in solid can be best represented as :
Structures of molecule of two compounds are given below:
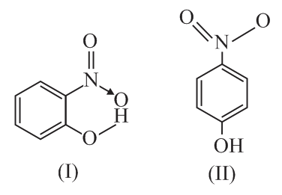
(a) Which of the two compounds will have intermolecular hydrogen bonding and which compound is expected to show intramolecular hydrogen bonding?
(b) The melting point of a compound depends on, among other things, the extent of hydrogen bonding. On this basis, explain which of the above two compounds will show higher melting point.
(c) Solubility of compounds in water depends on power to form hydrogen bonds with water. Which of the above compounds will form hydrogen bond with water easily and be more soluble in it?
Which of the following exhibit/s -bonding ?
The volatility of is low as compare to other Hydra acid of Halogen because of :-
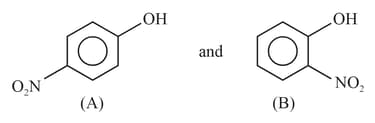
Out of the two compounds shown below, the vapour pressure of at a particular temperature is expected to be
Liquid does not contain -bond.
In which of the following substances will hydrogen bond be strongest?
Which of the following compounds would have significant intermolecular hydrogen bonding?
Explain why is a gas and is a liquid.
Hydrogen bonds are formed in many compounds e.g., , , . The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is:
Ethanol has a higher boiling point than dimethyl ether though they have the same molecular weight. This is due to:

