Electronic Effects
Important Questions on Electronic Effects
bond in (vinyl chloride) is stabilised in the same way as in:
The reason for resonance, is delocalisation of -electrons.
The number of groups showing effect .
The number of groups showing effect .
Find the value of , groups are given as follows :
(i) (ii) (iii) 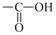 (iv)
(iv) 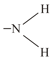 (v)
(v) 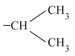 (vi)
(vi)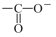 (vii) , (viii) (ix)
(vii) , (viii) (ix)
Stability of:
(I) 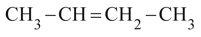
(II)
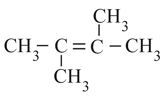
(III)
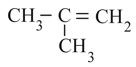
(IV)
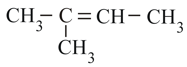
in the increasing order is:
Total number of resonating structures in the following cation.
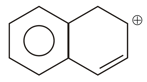
Most stable carbanion is
In which of the following molecule, all the effects namely inductive, mesomeric & hyper conjugation operate?
Formic acid is considered as a hybrid of the four structures
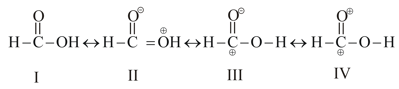
Which of the following order is correct for the stability of the four contributing structures.
Explain why ion cannot be represented by a single Lewis structure. How can it be best represented?
Which of the following shows the correct order of decreasing basicity in aqueous medium?
Which of the following shows the correct order of decreasing acidity?
Which of the following molecules has all the effects:
inductive, mesomeric and Baker Nathan effect?

