Relation Between Cp and Cv for an Ideal Gas
Important Questions on Relation Between Cp and Cv for an Ideal Gas
An ideal gas having the density at pressure is filled in a Kundt tube. When the gas is resonated at a frequency of , nodes are formed at a separation of . Calculate the molar heat capacities and of the gas.
Standing waves of frequency are produced in a tube filled with oxygen at . The separation between the consecutive nodes is . Calculate the specific heat capacities of the gas.
A mixture contains of helium and of hydrogen . Calculate the values of , and for the mixture.
Two ideal gases have the same value of . What will be the value of this ratio for a mixture of the two gases in the ratio ?
An ideal gas is taken through a process in which the pressure and the volume vary as . Find the value of for which the specific heat capacity in the process is zero.
An ideal gas is taken through a process in which the pressure and the volume are changed according to the equation . Show that the molar heat capacity of the gas for the process is given by .
An amount of heat is added to a monatomic ideal gas in a process in which the gas performs a work on its surrounding. Find the molar heat capacity for the process.
An ideal gas expands from to at a constant pressure of when of heat is supplied to it. Calculate
(a) the change in internal energy of the gas.
(b) the number of moles in the gas if the initial temperature is .
(c) the molar heat capacity at constant pressure.
(d) the molar heat capacity at constant volume.
A sample of air weighing occupies when kept at and . When of heat is added to it at constant volume, its temperature increases by . Calculate the amount of heat needed to increase the temperature of air by at constant pressure if the mechanical equivalent of heat is . Assume that air behaves as an ideal gas.
The specific heat capacities of hydrogen at constant volume and at constant pressure are and , respectively. The molecular weight of hydrogen is and the gas constant . Calculate the value of .
A rigid container of negligible heat capacity contains one mole of an ideal gas. The temperature of the gas increases by if of heat is added to it. The gas may be
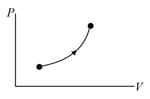
The molar heat capacity for the process shown in figure is
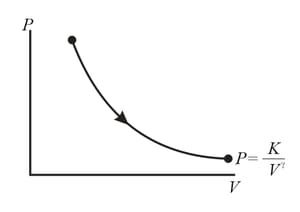
Figure shows a process on a gas in which pressure and volume both change. The molar heat capacity for this process is .
calories of heat is required to raise the temperature of of an ideal gas at constant pressure from to . The amount of heat required to raise the temperature of the same gas through the same range at constant volume is
Let and denote the molar heat capacities of an ideal gas at constant volume and constant pressure respectively. Which of the following is a universal constant?
The value of is for a gas sample in state and is in state . Let denote the pressure and and denote the temperatures of states and , respectively. Most likely
For a solid with a small expansion coefficient,
The ratio for a gas is . What is the degree of freedom of the molecules of this gas?
In a real gas the internal energy depends on temperature and also on volume. The energy increases when the gas expands isothermally. Looking into the derivation of , find whether will be more than , less than or equal to for a real gas.
Does a solid also have two kinds of molar heat capacities and ? If yes, do we have ? Is ?

