Variation Of Molar Conductivity With Concentration
Variation Of Molar Conductivity With Concentration: Overview
This topic elaborates on the variation of molar conductivity with concentration. It explains the Debye-Huckel and Onsager equation along with their relations and derivations. We will also study the applications of Kohlrausch’s law.
Important Questions on Variation Of Molar Conductivity With Concentration
Define limiting molar conductivity in terms of Kohlrausch law of independent migration of ions.
Define the term limiting molar conductivity. How can you determine limiting molar conductivity?
of an electrolyte offered a resiatance of in a conductivity cell having a cell constant of . Calculate the molar conductivity of the solution.
Give the mathematical equation which gives the variation of molar conductanctivity , with the molarity (c) of the solution.
The molar conductivity of methanoic acid is Calculate its degree of dissociation and dissociation constant. Given and
The following curve is obtained when molar conductivity, is plotted against the square root of concentration, along and -axis respectively for the two electrolytes and . Account for the increase in for the electrolytes and with dilution.
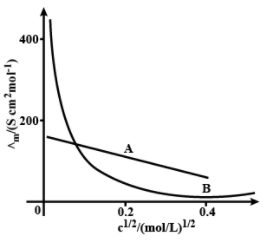
The molar conductance of 0.001 M acetic acid is . The maximum value of molar conductance of acetic acid is . What is the degree of dissociation of acetic acid ?
