Electrochemical Cell
Electrochemical Cell: Overview
This topic explains the concept of electrochemical cells and their types. It covers the details of the construction of a Daniel’s cell. The cathodic reduction, salt bridge, and emf of a cell are also illustrated here.
Important Questions on Electrochemical Cell
Explain the working of salt bridge.
Describe the construction and working of Daniell cell.
In a galvanic cell, the electrons flow from
The value of for metals , respectively. State the CORRECT order for their ability to act as reducing agent.
What does the negative sign in the expression mean?
Calculate the standard cell potentials of galvanic cell in which the following reactions take place:
Calculate and equilibrium constant of the reaction.(Given )
Depict the galvanic cell in which the reaction
takes place. Further show:
Individual reaction at each electrode.
The following is a sketch of an electrolytic cell used in the extraction of aluminium:
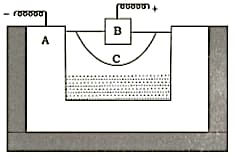
(i) What is the substance of which the electrodes and are made?
(ii) At which electrode (A or B) is the aluminium formed?
(iii) What are the two aluminium compounds in the electrolyte C?
(iv) Why is it necessary for electrode B to be continuously replaced?
Some word/words are missing in the following statement. You are required to rewrite the statement in the correct form using the appropriate word/words.
Cations migrate during electrolysis.
Give appropriate scientific reasons for the following statement:
During electrolysis of molten lead bromide, graphite anode is preferred to other electrodes.
Which electrode, anode, or cathode is the oxidising electrodes? Why?
Discuss briefly the function of the salt bridge in an electrochemical cell.
The standard EMF of the cell : is volt. The standard electrode potential (reduction potential) of copper electrode is volt. Calculate the standard electrode potential of nickel electrode.
Using the standard electrode potentials, predict if the reaction between the following is feasible:
and
, .
In which of the following electrochemical cell the overall cell reaction is :
