Hydrogen Atom Spectrum
Important Questions on Hydrogen Atom Spectrum
The wave number of the first emission line in the Balmer series of -Spectrum is:
( Rydberg's constant)
If the shortest of the hydrogen atom in the Lyman series is , what is the longest in the Balmer series of ?
The first emission line of the Balmer series in spectrum has the wave number in equal to:
( is the Rydberg's constant)
The electronic transition from to will produce the shortest wavelength in:
In spectrum, on one side of Brackett series, lies the Paschen series while on other side lies the Pfund series, then the second line of Brackett series towards Pfund end is:
Suppose that a hypothetical atom gives a red, green, blue & violet line spectrum. Which jump according to the figure would give off the red spectral line?
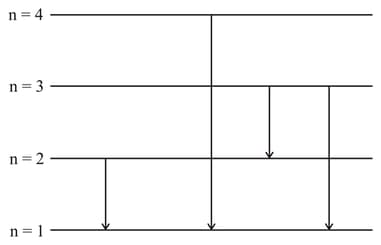
In a hydrogen atom, electron de excites from the third excited state to the ground state. What is the frequency of emitted radiation?
What is the wavelength of the series limit of Paschen series of -spectra?

