Embibe Experts Solutions for Chapter: States of Matter, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: States of Matter, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 5: States of Matter, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course BITSAT solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: States of Matter, Exercise 1: Exercise 1 with Hints & Solutions
The plot that is not valid for an ideal gas where is the pressure and is the volume of the gas is:
For an ideal gas, Boyle's law is best described by -
Two balloons, and , containing mole and mole of helium at room temperature and ., respectively, are connected. When an equilibrium is established, the final pressure of in the system is:
At , assuming ideal behaviour, the average kinetic energy of a deuterium molecule is:
Compressibility factor for behaving as real gas is:
At low pressures (For mole), the Van der Waal's equation is written as
.
The compressibility factor is then equal to:
The values of Van der Waals constant, , for the gases are , respectively. The gas which can most easily be liquefied is:
An ideal gas is subjected to a cyclic change as shown in the diagram below:
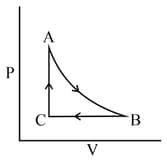
The step in which the gas will cool down is along:
