M K Verma Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: TOPICWISE QUESTIONS
M K Verma Chemistry Solutions for Exercise - M K Verma Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: TOPICWISE QUESTIONS
Attempt the practice questions on Chapter 1: General Principles and Processes of Isolation of Elements, Exercise 1: TOPICWISE QUESTIONS with hints and solutions to strengthen your understanding. Practice Book for MHT-CET Chemistry (Inorganic Chemistry 2) solutions are prepared by Experienced Embibe Experts.
Questions from M K Verma Solutions for Chapter: General Principles and Processes of Isolation of Elements, Exercise 1: TOPICWISE QUESTIONS with Hints & Solutions
In the froth flotation process, zinc sulphide and lead sulphide can be separated by:
(i) Using collectors
(ii) Adjusting the proportion of oil to water
(iii) Using froth stabilisers
(iv) Using depressants
In the following Ellingham diagram:
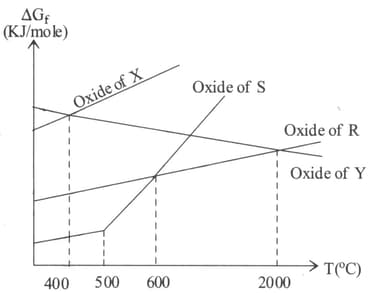
Free energy of formation, of and at and are given below.
The temperature at which carbon can be used as a reducing agent for is
If is plotted against then, it is a straight line:
passing through the origin with slope .
with intercepts and slope .
with intercepts and slope .
passing through the origin with slope .
A metal ore is a magnetic oxide ore. The metal obtained by its metallurgical process contains impurities that are less fusible than the metal itself. Which of the following processes might not have been employed in its extraction?
Choose the set of correct statements among the following.
Ellingham diagram is a plot between per mole of oxide formed versus temperature.
slope downwards; so, is used as a reducing agent for all the metal oxides.
Slag is easily fusible material that melts at the temperature of the furnace, soluble in the molten metal.
Hydrometallurgy involves the precipitation of ions from the solution in the presence of scrap.
Liquation is used when the melting point of the metal is lower than the impurities.
Choose the incorrect combination.
