Academic Experts Solutions for Chapter: Qualitative Analysis, Exercise 1: Exercise
Academic Experts Chemistry Solutions for Exercise - Academic Experts Solutions for Chapter: Qualitative Analysis, Exercise 1: Exercise
Attempt the practice questions on Chapter 7: Qualitative Analysis, Exercise 1: Exercise with hints and solutions to strengthen your understanding. Practice Book for KVPY Aptitude Test - Stream SX Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Academic Experts Solutions for Chapter: Qualitative Analysis, Exercise 1: Exercise with Hints & Solutions
A white crystalline solid on boiling with caustic soda solution gave a gas , which when passed through an alkaline solution of potassium mercuric iodide gave brown precipitate. The substance on heating gave a gas , which rekindled a glowing splinter but did not give brown fumes with nitric oxide. The gas is:
An aqueous solution of compound '' gives white precipitate with . The precipitate becomes black on addition of aqueous due to formation of ''. '' dissolves in aqua regia. '' and '' are
A compound reacts in the following ways.
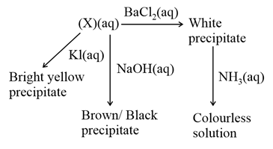
The compound is likely to be:
To a solution of a substance, gradual addition of ammonium hydroxide results in a brownish black precipitate which does not dissolve in excess of . However, when (not in excess) is added to the original solution, a green precipitate is formed. The solution contained:
Black precipitate of copper sulphide dissolves in
Which of the following metal salts gives a red and opaque borax bead in the reducing flame (in cold)?
Which one among the following pairs of ions cannot be separated by in dilute hydrochloric acid?
The reagents, and aqueous will precipitate:
