Embibe Experts Solutions for Chapter: Redox Reactions, Exercise 2: Level 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Redox Reactions, Exercise 2: Level 2
Attempt the practice questions on Chapter 20: Redox Reactions, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Main solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Redox Reactions, Exercise 2: Level 2 with Hints & Solutions
The resonating structures of the cyanate ion are 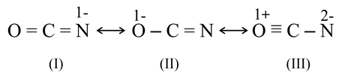 . The correct set of the oxidisation states of , respectively, with the most stable structure out of the above is:
. The correct set of the oxidisation states of , respectively, with the most stable structure out of the above is:
is oxidised by in the presence of an acid : . What are the whole number values of in that order?
In the following reaction:
Which of the following does not act both as an oxidising as well as reducing agent
If equal volumes of and solutions are allowed to oxidise to in acidic medium, then oxidised will be:
Which ordering of compounds is according to the decreasing order of the oxidation state of nitrogen?
Oxidation number of carbon in carbon suboxide is ____.
of anhydrous , present in a solution, was quantitatively converted into of . Find the equivalent weight of .
