Embibe Experts Solutions for Chapter: Structure of Atom, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Structure of Atom, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 2: Structure of Atom, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course KCET (UG) solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Structure of Atom, Exercise 1: Exercise 1 with Hints & Solutions
The graph that depicts Einstein's photoelectric effect for a monochromatic source of frequency above the threshold frequency is
A object is moving with a velocity of . The de-Broglie wavelength(in ) of the object is[Planck's constant, ]
The electronic configuration which obeys Hund's rule for the ground state of carbon atom is:
The maximum number of electrons that can be filled in the shell with the principal quantum number is:
The correct representation of wavelength intensity relationship of an ideal blackbody radiation at two different temperatures and is"
Among the following, the INCORRECT statement is
For a orbital, the number of radial and angular nodes, respectively, are:
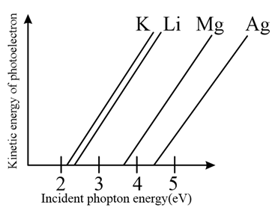
The photoelectric behaviour of and metals is shown in the plot below. If light of wavelength is incident on each of these metals, which of them will emit photoelectrons? [Planck's constant and ]